OVERACTIVE BLADDER STUDY
BlueWind Medical
Restore
Explore a new option for Urgent Bladder Leaks.
Customizable Revi System
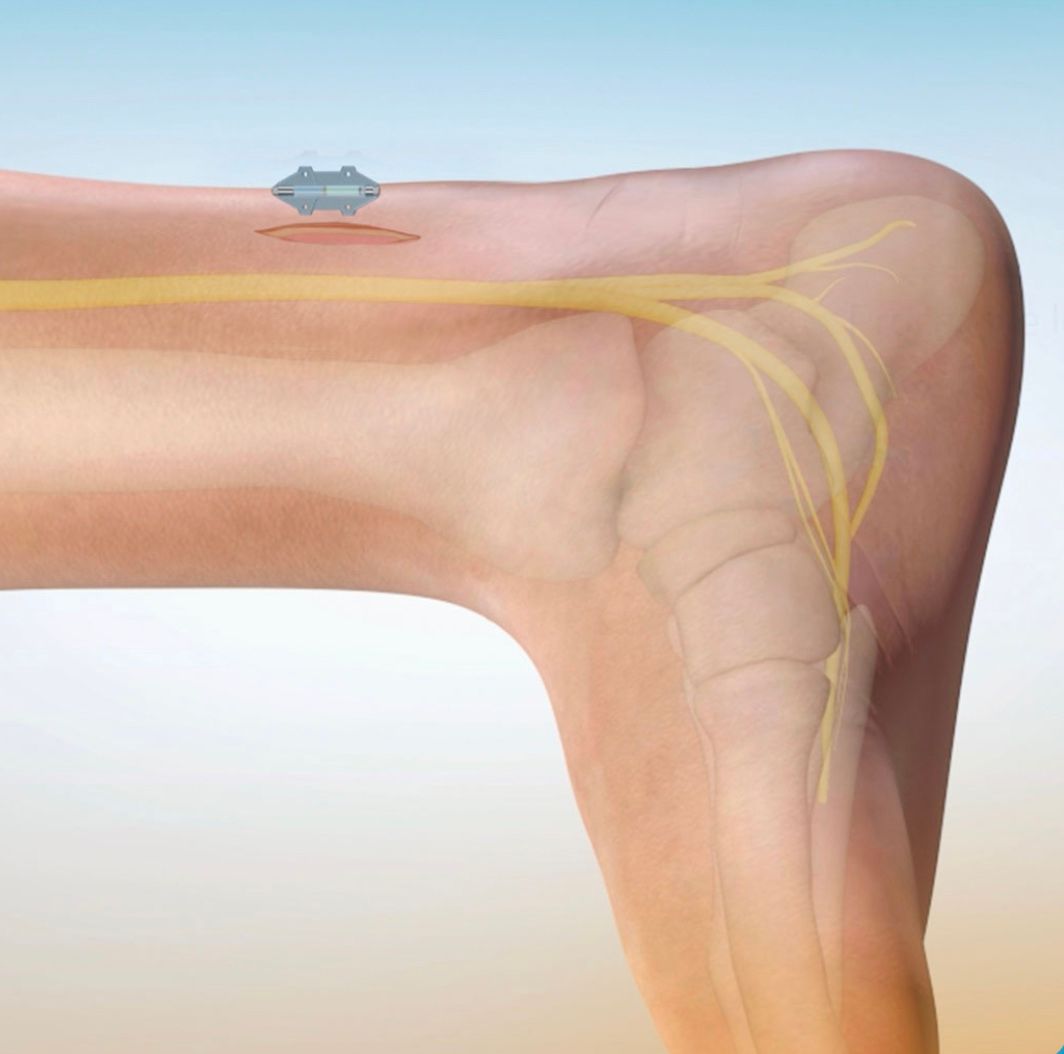
BlueWind Revi™ was crafted with the patient and the physicians who treat UUI in mind. This advanced implant design allows for the device to be miniature in size without the need for replacement surgeries for battery change, lead migration, or lead fracture. The innovative external wearable technology powers the implant for a patient-centric approach to therapy allowing for increases in energy and stimulation sessions based on your needs to ensure the best possible outcome.
Where Is The Implantation Done?
The procedure is completed in the operational room under anesthesia. The device will be implanted slightly above the ankle area as shown in the image above. The device is permanent and MRI-friendly. The implant does not create any risk or deviation to your daily life.
How Are Treatments Administered?
The treatment will be activated in the office however continuous treatments will be done at home. The device comes with a wearable cuff that will be placed around the ankle to administer treatment. The treatments are customizable and will be changed based on the provider's discretion at follow-up visits. Treatments are performed daily for about 30 minutes.
What Should I Expect?
Subjects will be asked to fill out questionnaires throughout the study and follow protocol procedures such as follow up appointments.
Interested in this Clinical Trial?
Email us at research@theupi.com for more information.
Join Our N
We will get back to you as soon as possible.
Please try again later.
Business Hours
- Mon - Fri
- -
- Sat - Sun
- Closed